There is an increasing interest in the dietary components of food and the
possible benefits of specific compounds on health and disease.
You may have been flooded with numerous advertising information encouraging
you to use curcumin “2017’s Top Turmeric curcumin: best anti-oxidant
and anti-inflammatory properties” or myo-inositol “Help regulates
cycles- naturally!” which left you wondering how does this work.
In this blog, we will describe the most recent studies showing how the
different ingredients used in our dietary supplement could
positively impact your oocyte quality and
improve the symptoms associated with PCOS and endometriosis.
In normal cells, metabolism produces reactive species that regulate diverse
cell functions.
In the ovary, reactive oxygen species (ROS) and reactive nitrogen species (RNS) are
produced by the developing oocyte (egg) and the surrounding granulosa
cells supporting its development (Figure 1). As a result, the follicular
fluid (FF) contains abundant levels of reactive species that are crucial
for regulating the ovarian function influencing the development of follicles,
the oocyte maturation, the production of steroids (1).
Nevertheless, these reactive species are highly reactive with other cell
components such as proteins, lipids or DNA (2) which could alter their
function and lead to potent
oxidative stress damaging the oocyte quality (3) by impacting DNA (4).
Mitochondria are small energy producing organelle that play a key role
in supporting the early step of embryo development. Moreover, oxidative
damage is much more prominent in mitochondria.
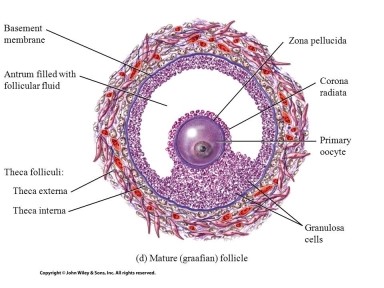
Figure 1: Mature follicle where the oocyte is surrounded
by granulosa cells and follicular fluid
1. Melatonin: Prime defense against oxidative and nitrosative stress.
Melatonin is by nature an agent able to pass through any cellular membranes.
It is a potent
anti-oxidant that prevents oxidative stress induced by reactive oxygen species. While
melatonin has a direct effect on oxidative stress by scavenging ROS and
RNS (5), its metabolites (product of its degradation) are also active
and act indirectly by inducing the production of anti-oxidative enzymes
and lowering the synthesis of pro-oxidative molecules (6)
leading to an overall reduction of oxidative stress.
In the FF, melatonin eliminates free radicals and stimulates antioxidant
enzymes in addition to promote sex steroid hormone production by granulosa
cells which
support follicle development leading to ovulation (7).
Melatonin plays also a key role during the luteal phase protecting the
luteal granulosa cells from oxidative damage leading to apoptosis and
preventing the occurrence of a premature new menstrual cycle. It maintains
progesterone production by the corpus luteum which supports the luteal phase.
There are many clinical studies assessing the use of melatonin during IVF
cycles although most of them have been conducted with patients as their
own controls and not through a randomized, double blinded comparison between
treated patients “melatonin group” and proper controls “placebo
group”.
A study (8) assessing the effects of oral administration of melatonin (3mg/day)
in patients from day 5 until oocyte collection showed significant
increase in fertilization rates as compared to the precedent cycle for the same patients (50% vs 20.2%).
A small randomized study (9) with N=60 patients where melatonin was orally
administrated (3mg/day) from day 3-5 until hCG administration (oocyte
collection) showed:
- a higher number of total oocytes (11.5 vs 6.9)
- higher number of mature oocyte (9% vs 4.4%)
- a higher rate of embryos transferred (69.3% vs 44.8%)
These results were confirmed in another independent trial (10).
Animal studies showed that melatonin can reduce endometriotic lesions (11)
although it has not been assessed in women.
Nevertheless, a recent study has shown promising results in PCOS patients
with restoration of menstrual cyclicity (lowering testosterone and AMH
levels, increasing FSH with no alteration of glycemic or lipid parameters)
when administrated at 2mg/day for 6 months (12).
Melatonin use is
safe and did not show any teratogenic effects in both human and animal (13-14)
as well as no toxicity (15) even at very high dose (5-20mg/day).
Given the potential clinical benefits of melatonin and its safety, it
is a target of choice
to counteract oxidative stress and enhance oocyte/embryo quality.
2. Myo-inositol: optimizer of the ovarian function
Myo-inositol belongs to the vitamin B complex. It is the precursor for
the synthesis of phosphoinosides, which are part of the phosphatidylinositol
signal transduction pathway (16). This pathway is responsible of signal
transduction across the plasma membrane, via a second messenger, inositol
1,4,5-triphosphate, that
modulates intracellular Ca2+ release as seen in Figure 2.
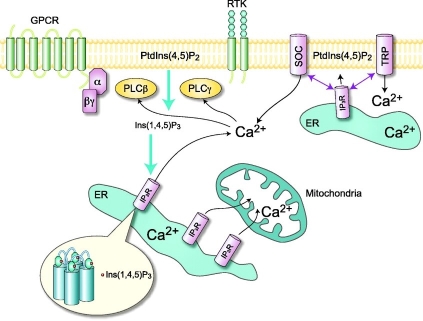
Figure 2: Phosphatidyl inositol pathway leading to calcium release.
Myo-inositol and the ovarian function
Through its effects on the calcium pathway, myo-inositol plays a
direct and key role on the maturation of an oocyte (egg) which leads to
better oocyte quality and increased ovulation rate (17-19). Further this has an
impact on fertilization and early embryo development (20).
Thus, myo-inositol significantly
increases fertilization and pregnancy success rate (21) which is suggested by its role during the stages of early development
in the embryo (22).
Indeed, a double blinded trial (17) assessed the effects of myo-inositol
on the ovarian function by comparing PCOS patients on 2g MYO + 200mcg
folic acid (twice a day) for 3 months (treated group) versus PCOS patients
on 200mcg folic acid (twice a day) for the same period length (control group).
The oocyte quality was assessed at ovum pick up and showed:
- rFSH administrated during stimulation was significantly lower in the treated
group which significantly decreases the risk of hyperstimulation.
- Increased number of oocytes retrieved in the treated group (12 versus 8.5)
- Higher % of mature oocyte in the treated group (82% versus 63%)
- % of immature oocyte (degenerated or vesicle germinal) was significantly
reduced in the treated group (2.3% versus 24%)
- Higher % of score 1 embryos (good quality embryo) in the treated group
(70% vs. 30%).
The treatment with myo-inositol in addition to melatonin improves ovarian
stimulation protocols and pregnancy outcomes in infertile women with poor
oocyte quality (21).
It is important to also highlight that levels of myo-inositol in the follicular
fluid are good marker of oocyte quality in animal study (23) but also
in women during IVF procedure (24). Further, myo-inositol is also able
to significantly lower leptin levels that are markers for poor oocyte
quality (25).
Myoinositol and PCOS
Another important aspect of inositols is their role as
insulin-sensitizers that could have several benefits in PCOS patients.
Besides their powerful antioxidant potential (26) that reduces oxidative
stress, myo-inositol can also help decrease hyperandrogenism (lowering
testosterone levels) and sensitize the ovary to insulin (27).
Further, a study assessing hormonal parameters in obese PCOS patients before
and after 12 weeks of treatment (28) with myo-inositol at 2g/day in addition
to folic acid (200mg) and comparing the changes to control individuals
(treated with folic acid 200mg only) showed exclusively in patients treated
with myo-inositol:
- A significant reduction in insulin levels
- A significant reduction in prolactin levels
- A significant reduction in LH levels
In addition, myo-inositol had a clear impact on treated patients’
fertility who had much higher % of top-quality oocytes (82% vs. 36%) resulting
in 40% of clinical pregnancies (vs 16% for the control group).
Myo-inositol supplementation is efficient in positively modulating many
of the hormonal disturbances of PCOS, and overall leading to
better ovarian function.
It could be used as an agent to help
restore better oocyte quality leading tobetter embryo quality and higher chances of getting pregnant.
3. Curcumin
Curcumin (diferuloylmethane) is the golden spice in Indian saffron and
the most active constituent of turmeric. It has been consumed by people
for centuries to treat a variety of proinflammatory ailments.
It has been shown to have high anti-oxidant potential (29). It also has
anti-inflammatory properties and has been shown as a valuable agent to
prevent inflammatory damage in a model of intestinal disease (30) or in
a model of chronic kidney disease (31).
Studies led in animal models showed a positive impact of curcumin on ovarian function.
A mice model of immune ovarian failure (32) where, a factor NF-kappa B
leading to pro-inflammatory factors secretion, triggers the follicular
cell death, curcumin has been shown to:
- attenuate the inflammatory response.
- promote the proliferation of granulosa cells by reducing the apoptosis
process leading to cell death
- support the oocyte maturation that was impaired in this mice model
- promote the synthesis of sex steroid hormones
Other in-vivo studies have demonstrated the benefits of curcumin in supporting
follicle development and steroidogenesis (33-35).
Curcumin has shown some promising results with potential therapeutic use
in the prevention and treatment of endometriosis.
An in vitro study (36), where human ectopic endometriotic cells were cultured
with curcumin, showed a
significant inhibition of TNF-α-induced secretion of
IL-6, IL-8 and MCP-1, known actors in the development of endometriosis. Further, curcumin inhibited
the expression of NF-kappa B, a master regulator of inflammation.
Another study in vitro showed that curcumin could
arrest endometriotic cells proliferation in a dose-dependent manner by repressing the activity of matrix metalloproteinase-9
(MMP-9), a factor involved in tissue remodeling.
Curcumin could be used as a
new line of therapeutic intervention to improve the ovarian function and
counteract endometriosis development.
4. CoQ10
A natural fertility enhancer
The maturation of an oocyte leading to ovulation is a complex process requiring
the formation of the meiotic spindle and the
production of energy by mitochondria (38).
A disrupted mitochondrial function could lead to arrest of oocyte maturation,
chromosomal misalignment, and could compromise embryo development (39-41).
CoQ10 is an essential component of the electron transport chain involved
in energy (ATP) production (42), further it has critical anti-oxidant
properties (43).
A murine model for ovarian aging showed that mitochondria are not fully
functional in aged ovaries as seen by decreased metabolic activities (44).
As a result, aged animals produced oocytes with spindle defects leading
to misaligned chromosomes (that will induce impaired cell division).
Interestingly,
most of these abnormalities could be partially or completely corrected
by the administration of CoQ10
in animal models.
Besides changes in the mitochondria, it has been shown that older animals
have lower number of granulosa cells (GC, cells surrounding the oocyte
and supporting the growth of the oocyte) and these cells express lower
CoQ10 expression levels.
CoQ10 treatment significantly increases GC numbers in these aged mice (44).
In the same study, the genetic deletion in the oocyte of a specific enzyme
Pdss2 (leading to its inactivation), involved in the CoQ10 synthesis led to aged
oocytes in young mice with:
- Premature ovarian failure
- Reduction of follicle number
- Poor ovulation response to stimulation
- Decreased ATP production and mitochondrial activity in the few aged oocytes.
Altogether, these data suggest that a
lack of CoQ10 could be responsible for a
premature aging of the ovarian function with
detrimental effects on oocyte quality.
Most interestingly, CoQ10 supplementation could be
used to counteract and even reverse the effects of aging on follicle development.
Indeed, a clinical trial has been led in women and showed interesting results.
In the randomized, double-blind-study including IVF-ICSI patients between
35-43 years old, women were treated with either 600mg CoQ10 (N=17) or
equivalent dose of placebo (N=22) for 2 months prior and during their
IVF cycle (45).
The rate of oocyte aneuploidy (using polar body biopsies) was 46.5% in
the CoQ10 group compared to 62.8% in the controls. Clinical pregnancy
rate was 33% for the CoQ10 group and 26.7% for the control group. Although,
the difference was not significant due to the limited number of patients
enrolled in the study, the data showed a
trend towards oocyte improvement in CoQ10 treated patients.
In addition, levels of CoQ10 in the follicular fluid of N=20 infertile
women correlate with oocyte maturation and embryo grade during in vitro
fertilization (46).
Another study (47) on N=60 patients undergoing ICSI showed that higher
follicular fluid CoQ10 level were associated with grade A-B embryos (0.53
µg/mL) as compared to grade C-D embryos (0.39 µg/mL).
Further, embryos leading to pregnancy were obtained from oocyte with higher
follicular CoQ10 levels (0.6 µg/mL) when compared to those leading
to failed implantation (0.38 µg/mL).
Other studies have shown that low plasma CoQ10 levels correlates with
subsequent spontaneous abortions (48).
CoQ10 and PCOS
Besides its beneficial effects on fertility, CoQ10 could also improve glucose
metabolism and lipid profile in PCOS patients (49). A randomize, double-blind,
placebo controlled trial where patients were administrated with 100mg
CoQ10 daily (N=30) or placebo (N=30) for 12 weeks showed that treated
patients have:
- Significantly lower fasting plasma glucose
- Significantly lower serum insulin concentration
- Significantly lower total cholesterol concentration
- Significantly lower LDL-cholesterol concentration
So Overall, CoQ10 supplementation for 12 weeks among subjects with PCOS
had beneficial effects on glucose metabolism, serum total- and LDL-cholesterol levels.
In PCOS patients (50) resistant to clomiphene citrate induction, CoQ10
could induce ovulation when combined to clomiphene citrate (N=51, 65.9%
ovulation/cycle) as compared to controls (N=50 patients treated with clomiphene
citrate alone, 15.5% ovulation/cycle) which leads to higher pregnancy
rate per patient (37.3% vs 6%).
Lastly, CoQ10 reduces gamma glutamyltransferase, a specific enzyme involves
in oxidative stress within 14 days of 150mg/day CoQ10 administration.
Altogether, these results are supportive of the usage of CoQ10 as a supplement
in women fertility.
5. Pine tree Bark: pycnogenol
Pycnogenol® (PYC) is a plant extract obtained from the bark of the
French maritime pine Pinus pinaster. It has strong antioxidant activity
and is used as a phytochemical remedy for various diseases.
In Vitro study in human lymphocytes showed its potent anti-oxidant potential as
well as its abilities to reduce DNA damage and chromosome breakage induced
by chemicals (51).
Pycnogenol has been shown to exert anti-inflammatory and antithrombotic
effects (52) by inhibiting Cox-1 and Cox-2 enzymatic activity (53), both
involved in the inflammatory pathway.
PYC is also able to significantly reduce pain associated with endometriosis,
which is even reduced when combined with oral contraceptives after 3-months
use (54). These effects are mediated through the suppression of NF-KB-dependent
gene expression, which activates the inflammatory cascade (55).
In a study including N=58 patients affected by endometriosis and surgically
diagnosed with the condition, the use of pycnogenol at 60mg/day for 48
weeks significantly reduced the symptoms score (N=26) as well as CA-125
levels (a serum marker of endometriosis) although in patients treated
with hormones therapy (N=26), the benefits are even more pronounced.
6. Resveratrol
Resveratrol is a polyphenolic compound isolated from the skin of red grapes
and berries and found in red wine. In addition to its anti-inflammatory
properties, it is a natural aromatase inhibitor (57), an enzyme involved
in the synthesis of estradiol and has also antiproliferative and anti-oxidant
properties (58).
Resveratrol and the ovarian function
In the ovary, resveratrol can activate a specific receptor and increases
its expression, namely SIRT1 (59), present in oocyte and granulosa cells
at different stages of the follicular development. The sirtuin pathway
plays a key role in promoting mitochondrial biogenesis (60). This pathway
is also involved in:
- sensoring the oxidative stress levels in oocyte and granulosa cells
- activating the steroidogenesis associated with luteinization (progesterone
production)
- repressing the pro-inflammatory NF-KB pathway
- repressing the synthesis of COX enzyme involved in prostaglandins production
(pro-inflammatory pathway)
A recent mice study (61) showed that
resveratrol promotes ovary and oocyte quality by interfering with a pesticide (mancozeb) used to induce accumulations
of ROS (marker of oxidative stress). As a result, the abnormal mitochondrial
function, the increased follicle apoptosis, the decreased development
of mature oocyte is significantly minimized using resveratrol thus improving
the reproductive outcomes. Similar effects were also reported in porcine
oocytes cultured with resveratrol where mitochondrial functions including
ATP generation were improved as well as the developmental ability of the
oocytes to the blastocyst stages (62).
In addition, resveratrol increased the ovarian follicular reserve and
prolonged the ovarian life span in rats (63) by increasing AMH levels
and reducing ovarian inflammation through SIRT1 regulation among other
mechanisms, leading to the inhibition of the pro-inflammatory NF-KB pathway.
Resveratrol and endometriosis
A recent and elegant study revealed that infertility related to endometriosis
may be due to oocyte (egg) DNA damages induced by oxidative stress (64).
Most importantly, the study further suggested that
oxidative stress (the main cause of oocyte DNA damage) could be reversed
using anti-oxidants such as resveratrol and melatonin thus rescuing the oocyte and leading to its development and maturation
to give rise to a
fertilizable egg.
To assess the effects of oxidative stress on oocyte development, the authors
of the study exposed
in vitro, immature healthy mouse oocyte to follicular fluid from women affected
by endometriosis (ENDO-FF). They monitored the oocyte development and
compared it to immature mouse oocyte cultured with follicular fluid from
healthy women (controls patients not affected by endometriosis).
Results showed that Endo-FF induced:
- Higher levels of ROS in the mouse oocyte
- Higher levels of DNA damage in the mouse oocyte
- Decrease or a delay in oocyte maturation as compared to controls
Oocyte maturation was impaired by Endo-FF and the oocytes’ development
was blocked.
Interestingly, the oocyte maturation was blocked at a very specific development
stage (metaphase I arrest) where a
sensor for DNA damage (Spindle Assembly Check point/Dna Damage Response) assesses the DNA integrity
and blocks the oocyte maturation through the activation of a protein (ATM kinase).
The study showed that
ROS directly activates this blocking protein to stop oocyte development.
Further, the authors showed that
oocyte maturation could be rescued by inhibiting or lowering ROS levels using
resveratrol and melatonin in the culture medium.
It is very important to note that the noxious effects of ROS and pro-inflammatory
factors presents in the follicular fluid of ENDO patients (such as IL-6,
TNF-α) would be
even more accentuated
in human ovary where oocytes are exposed to follicular fluid at higher concentrations
and for longer periods of time than in the current study.
In a mice model for endometriosis (65), endometriotic human implants were
injected in the peritoneal cavity. Mice were treated by estradiol alone
daily for 12 days (controls) or in combination with resveratrol daily
for 20 days (controls). Mice were sacrificed and endometriotic lesions
were assessed.
Results showed a
60% reduction in the number of lesions and an
80% reduction in the lesions’ volume in mice treated with resveratrol as compared to the control group (estradiol
only). In the same study,
in vitro investigations showed that resveratrol significantly
reduce the invasiveness potential of human endometrial cells by up to 78%.
Besides confirming these results, another study run in rats (66-67) showed
a significant decrease in pro-angiogenic factors such as VEGF at both
serum and peritoneal fluid levels, but also a significant decrease in
pro-inflammatory molecules such as MCP-1 in the peritoneal fluid and at
the serum level. In addition, resveratrol-treated rats showed endometriotic
lesions improvement (decreased number and volume) with significant decrease
in oxidative stress (68) as shown by reduced activities of superoxide
dismutase and glutathione peroxidase (two enzymes involved in the generation
of oxidative stress).
Based on animal and
In Vitro studies (69),
resveratrol appears to be
effective in counteracting the development of endometriosis through its
antiangiogenic, anti-inflammatory properties, inhibiting the adhesion
and proliferation of endometriotic lesions and reducing the oxidative stress.
Resveratrol and PCOS
The therapeutic potential of resveratrol in the treatment of PCOS has been
postulated because this polyphenol promotes apoptosis (70) and reduces
androgen synthesis (71) in ovarian theca cells.
A mice model for PCOS has been developed (ob/ob mice that are also leptin
deficient), where mice are:
- obese
- hyperglycemic
- hyperinsulinemic
- insulin-resistant
- exhibiting increased TNF-a and IL-6 levels, which promotes the state
of chronic low-grade inflammation.
A study (72) assessing the effects of resveratrol on metabolic parameters
in ob/ob mice injected daily with resveratrol for 20 days or left untreated
(as controls) showed that resveratrol:
- reduces testosterone
- reduces insulin levels
- reduced TNF-α and IL-6 levels in adipose tissue
In a rat model for PCOS (73), daily intraperitoneal injection of resveratrol
for 4 weeks induced:
- a significant reduction in the number of antral follicle counts
- a significantly decreased plasma anti-Mullerian hormone and insulin-like
growth factor 1 levels (playing a key role in PCOS metabolic disturbances)
- significantly lower superoxide dismutase activity
- significantly increased glutathione peroxidase which will reduce cell vulnerability to ROS.
Altogether these data showed that resveratrol is effective in the treatment
of PCOS through its antioxidant properties.
Reducing oxidative stress (ROS and free-radicals) in the oocyte is of prime
clinical importance when treating sub-fertility in
endometriosis patients or in any patients whose oocyte quality is affected by high levels of oxidative
stress such as in
PCOS or Correctible Reoccurring Aneuploid Conversion Syndrome or
CRACS patients (For more information,
read our blog on CRACS).
As pioneer in the field of Reproductive Immunology, it is with the greatest care, that we studied any scientific evidences
showing beneficial effects of these different ingredients on women fertility.
The Endo-Optimize supplement is the result of our work and has been developed
to be the best solution to counteract oxidative stress induced-DNA damage
and optimize your egg quality as well as minimizing your symptoms associated
with endometriosis and PCOS.
This
all in one pill contains many ingredients
enhancing
mitochondrial activity (a key component in oocyte development) and reducing inflammation thus allowing
optimal microenvironment for the oocyte development and maturation.
References