Laparoscopic excision of visible endometriosis + laser destruction of the peritoneum including the cul de sac significantly increases
your chances of having a successful pregnancy.
We have studied two surgical approaches in our patients with systemic evidence of endometriosis as identified by our complete immunology profiles and fertility histories. Many of whom had no physical symptoms of endometriosis (read our blog on Silent Endometriosis)
The FIRST one focused on what is visible and removes only the endometriotic lesions by excision, we will name this approach endometriotic lesion excision or ELE.
The SECOND approach is more extensive and in addition to ELE, the peritoneum is destroyed by laser after excision of all visible lesions with focus on the anterior and posterior cul de sac regardless of the presence of visible lesions.
To simplify the graphs presented below, we will name this new approach ELE+ PD (endometriosis lesions excision + peritoneum destruction).
By analyzing the pregnancy outcomes in patients undergoing surgical laparoscopy, we noticed a significant and very marked difference between the two groups (ELE vs. ELE +PD).
By analyzing the pregnancy outcomes in patients undergoing surgical laparoscopy, we noticed a significant and very marked difference between the two groups (ELE vs. ELE +PD).
Figure 1: Pregnancy success rate in patient post laparoscopy. Significance is indicated by the p value, * means p-value<.05, ** means p-value <.01, *** means p-value <.001.
When patients conceived post ELE, they have 60.8% of chances of maintaining the pregnancy and being successful with the delivery of a baby (stated as Pregnancy Success in the graph, green bar).
This percentage increased to almost 82% (blue bar) when the patient conceived post ELE+PD as seen in Figure 1.
The presence of 3 stars indicates a highly significant finding with a P value < .001.
The rate of miscarriages is more than 2 times lower in ELE+PD patients (blue bar) as compared to ELE patients (green bar). The presence of 2 stars indicates a highly significant finding with a P value < .01
This clearly shows the benefit of peritoneal destruction including the cul de sac in addition to removing the visible endometriotic lesions.
The cul de sac or pouch of Douglas (Figure 2) is an extension of the peritoneal cavity:
Before going further in explaining how peritoneal destruction (laser destruction of the cul de sac) may give you better chances for pregnancy success, it is important to remind you the different theories of endometriosis as discussed in the section 1 below.
In the third section, we will explain you why the endometriotic lesions excision only may be less effective in lowering your risk for endometriosis reoccurrence as compared to procedure including peritoneal destruction as seen in Section 4. Lastly, we will discuss how peritoneal destruction may modulate immune regulation thus positively impacting your chances for a successful pregnancy.
were monitored post-surgery for conception
If we consider the implantation rate (positive βhCG post transfer or after spontaneous conception),
our surgical approaches in addition to our immune therapy protocol are
more efficient in allowing patients to get pregnant as seen in the Figure 4 below.
Our implantation rate (ELE only in addition to immune therapies or ELE+PD in addition to immune therapies )
are significantly higher (2.5-fold) than the one reported in Alkudmani’s study where lesions were lasered by CO2 vaporization (37) or in Slabuszewska’ study (38) where lesions ablated were present in the recto-vaginal area.
Besides, the beneficial effects of our surgeries in restoring a more tolerogenic environment, the higher
implantation rate in our patients could be attributed to our immune therapies and our dietary supplements providing a
better support to egg and embryo quality by reducing inflammation, improving mitochondrial egg
function and reducing oxidative stress, all dramatically impacting chances of being successful.
*** means that the p-value is <.001 and the difference seen between our surgical approaches in addition to
our immune therapies and the published studies is highly significant.
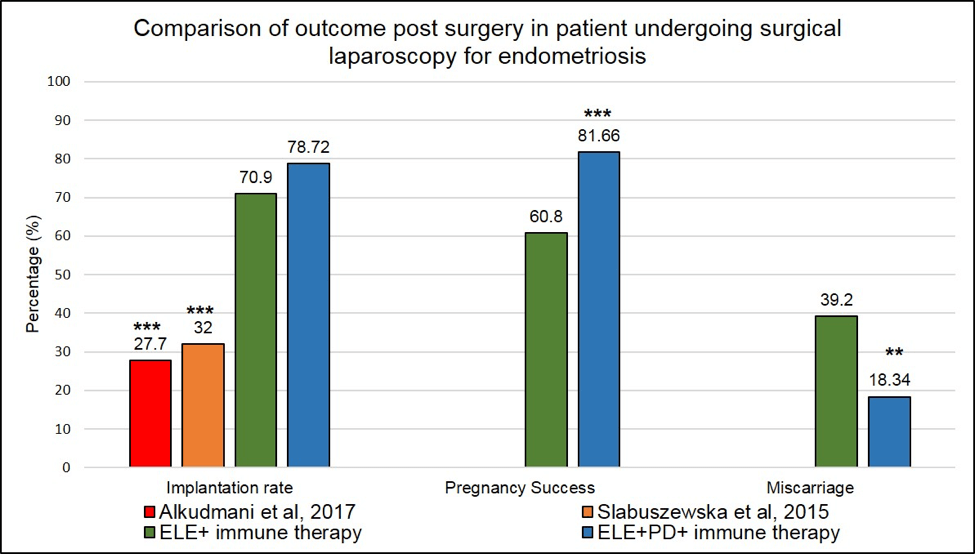
Figure 4:Outcome post-surgical laparoscopy in endometriosis patients. Comparison between our surgical approached in addition to our immune therapies and published studies. *** means that the p-value <.001
The second step of our study was to look for immunologic differences seen in pregnancy success and failures in relation to the two different surgical approaches.
When considering all our patients, regardless of underlying issues (autoimmune diseases, PCOS, endometriosis), we know that some immune parameters are modulated during pregnancy and whose expression levels correlate with the outcome of a pregnancy (analyze of N=278 pregnancies).
We have identified several IL-17 producing cells whose dysregulation correlated with miscarriages. As seen in Figure 5 and Figure 6, a peak of expression at 6-9 week of pregnancy in CD3+CD8+ IL-17 producing cells (Tc17) and CD3-CD56+IL-17 producing cells (NK-IL-17) is associated with miscarriages (red line is significantly higher=miscarriage as compared to blue line= pregnancy success, * means p-value <.05)
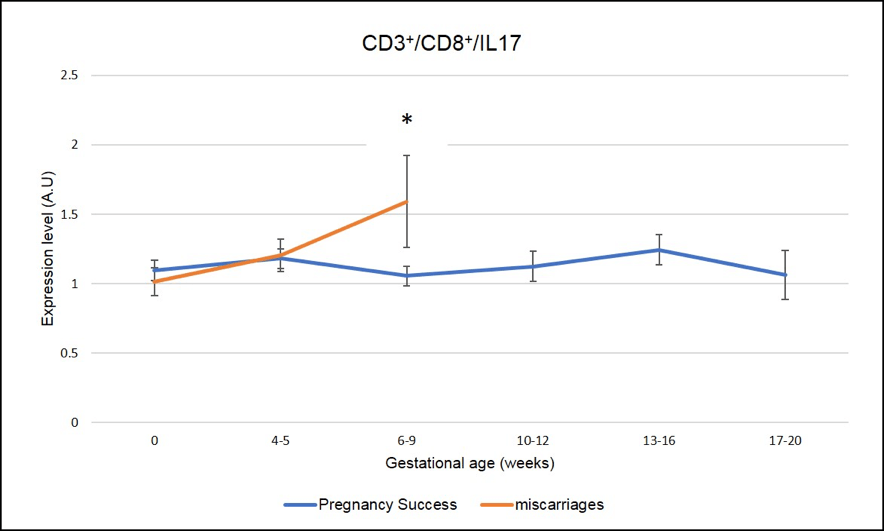
Figure 5: Miscarriages are associated with a pick of CD3+CD8+ IL-17 producing cells (Tc17) on week 6-9 of pregnancy. * means that the p-value is <.05 which indicates a significant difference
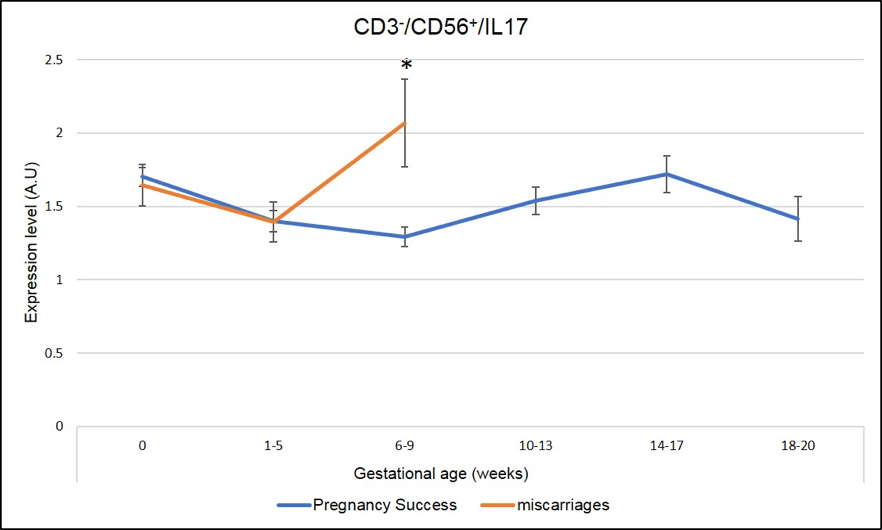
Figure 6: Miscarriages are associated with a pick of CD3-CD56+ IL-17 producing cells (NK-IL-17) on week 6-9 of pregnancy. * Means that the p-value is <.05 which indicates a significant difference.
In addition, and specifically in ENDO patients (N=219 pregnancies analyzed), a significant increase in CD3+CD4+IL-17 (Th17) also correlates with miscarriages at 6-9 weeks of pregnancy as seen in Figure 7 (the red line is higher than the blue line, * means that the difference seen is significant with a p-value <.05).
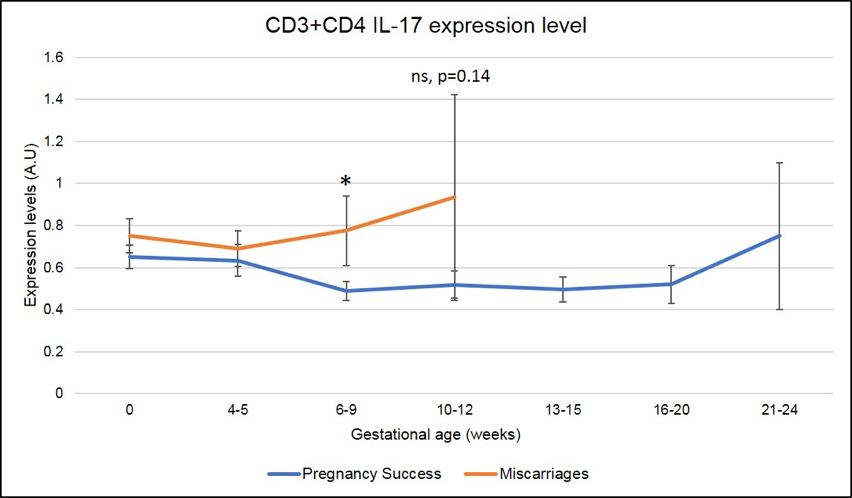
Figure 7: Miscarriages are associated with a pick of CD3+CD4+ IL-17 producing cells (Th17) on week 6-9 of pregnancy. * means that the p-value is <.05 which indicates a significant difference.
Focusing on these three parameters, Tc17, NK-17 and Th17, we wanted to determine if the surgical technique ELE or ELE+PD differentially alters the immune profile during the early stages of pregnancy.
In other words, can the peritoneum destruction place a patient in a more tolerogenic immune profile thus allowing the progression of a pregnancy?
To this end, we compared the immune profile across the different stages of pregnancy (including successful pregnancy with progression to term and pregnancies that will end up with a miscarriage) based on the surgical approach: ELE with PD (N=47 pregnancies) or ELE without PD (N=142 pregnancies).
Strikingly, all markers of pregnancy losses, namely Tc17 (Figure 8), NK17 (Figure 9) and Th17 (Figure 10) are significantly decreased in pregnancy achieved post ELE+PD (red line) as compared to ELE only (blue line).
* means that the p-value is <.05 which indicates a significant difference.
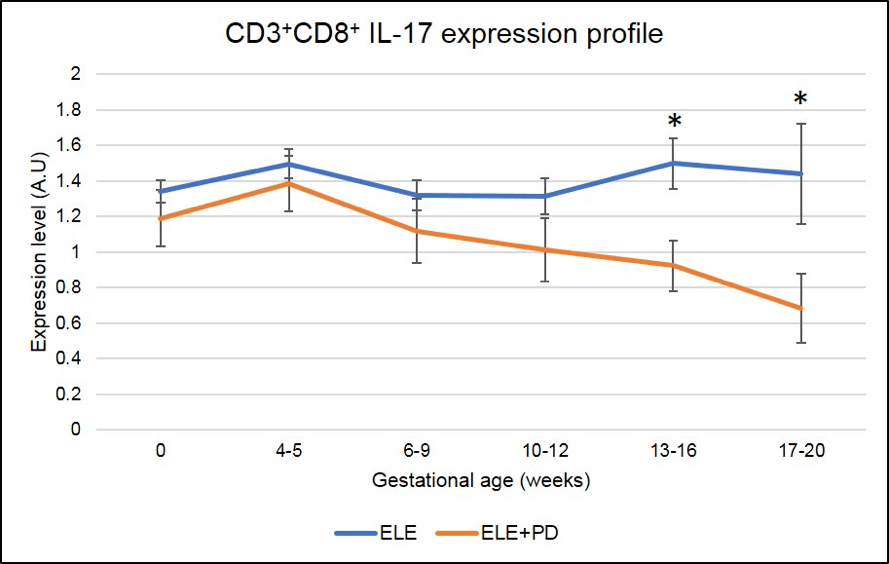
Figure 8: CD3+CD8+ IL-17 producing cells (Tc17) expression is significantly lower in pregnancy after ELE+PD as compared to ELE only. * means that the p-value is <.05 which indicates a significant difference.
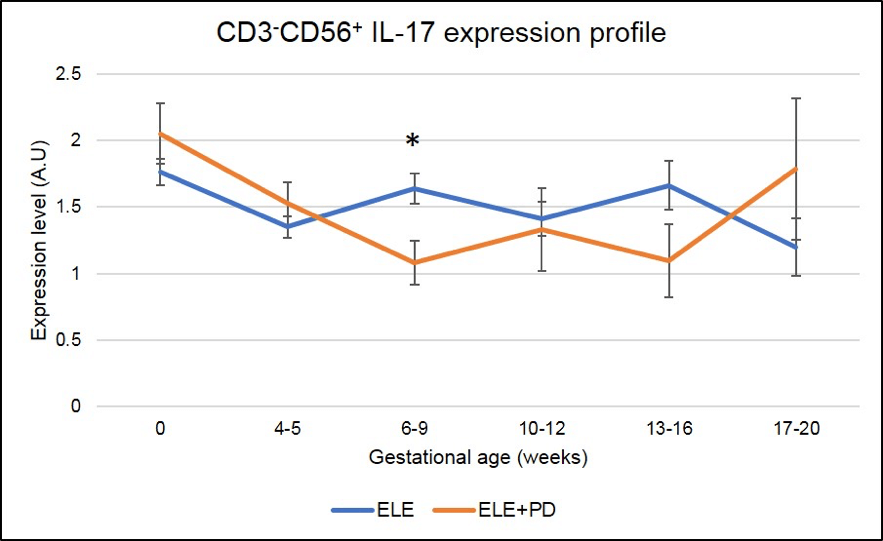
Figure 9:
CD3-CD56+ IL-17 producing cells (NK17) expression is significantly lower in pregnancy after ELE+PD as compared to ELE only. * means that the p-value is <.05 which indicates a significant difference.
Most importantly, Th17 cells expression, subset of cells known to contribute to the pathogenesis of endometriosis (37) and found to be strongly associated with miscarriages in our ENDO population, is dramatically reduced in pregnancies achieved post ELE + PD as seen in the Figure 9 below (the red line ELE+PD is strongly and significantly reduced as compared to the blue one representing pregnancies achieved post ELE only).
This puts into light the fact that pregnancies post ELE + PD are most likely to succeed than pregnancies achieved post ELE only, as seen by the significantly higher rate of pregnancy success in Figure 1 (i.e 82% vs. 60%).
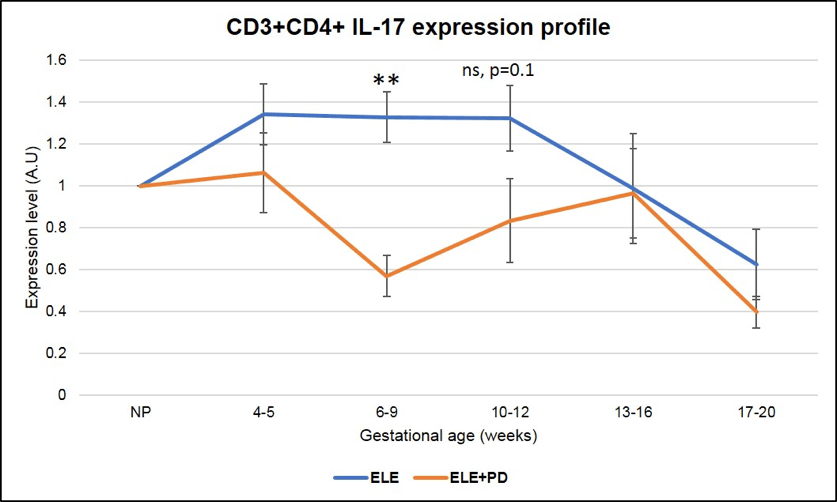
Figure 9:
CD3+CD4+ IL-17 producing cells (Th17) expression is significantly lower in pregnancy after ELE+PD as compared to ELE only. ** means that the p-value is <.01 which indicates a highly significant difference.
Peritoneal destruction induces a more tolerogenic uterine environment when combined with endometrial lesion excision resulting in a higher successful pregnancy rate.
It is now our recommendation that those patients with Immunologic and clinical evidence for endometriosis, even if MINIMAL or NO LESIONS are identified, undergo ELE+ PD.
Therefore, some of our patients that have had only excision of endometriosis without PD and continue to fail fertility treatments, are now recommended to repeat a laparoscopic surgery to include PD.
Although the number of cases is limited, we have seen improvement in these patients’ success rates as well. (there are not enough cases yet to validate this claim and this part of the study is ongoing but looks promising).
Endometriosis is a complex disease where genetic, environmental factors, diet and inflammatory disorders can play a role in the initiation and progression of the disease.
There is no simple solution to this very complicated disorder and at Braverman Reproductive Immunology, we offer a full program to counteract the deleterious effects of endometriosis on our patient’s fertility.
This ranges from laparoscopic surgery with lesions excision and peritoneal destruction to immune therapy to minimize and control the inflammation during pregnancy.
In addition, we have developed two supplements Endo-Optimize supplement and Endo-Optimize probiotic that could help in restoring your egg mitochondrial function, for more information read our blog on the topic. These two supplements may help improve your egg and embryo quality by reducing inflammation, oxidative stress and its associated DNA damage.
Further, many of the ingredients included in our supplements may have beneficial impact on endometriosis. For more information, read our blog.
References:
1- Buck Louis, G.M., Hediger, M.L., Peterson, C.M., Croughan, M., Sundaram, R., Stanford, J., Chen, Z., Fujimoto, V.Y., Varner, M.W., Trumble, A., Giudice, L.C., 2011. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil. Steril. 96, 360–365.
2- Giudice, L.C., Kao, L.C., 2004. Endometriosis. Lancet 364, 1789–1799.
3- Rawson JM. Prevalence of endometriosis in asymptomatic women. JRM 1991; 36:513–5.
4- I. E. Sasson and H. S. Taylor, “Stem cells and the pathogenesis of endometriosis,” Annals of the New York Academy of Sciences, vol. 1127, pp. 106–115, 2008.
5- P. R. Koninckx, S. H. Kennedy, and D. H. Barlow, “Endometriotic disease: the role of peritoneal fluid,” Human Reproduction Update, vol. 4, no. 5, pp. 741–751, 1998.
6- Bidarmaghz B, Shekhar A, Hendahewa R. Sigmoid endometriosis in a post-menopausal woman leading to acute large bowel obstruction: A case report. Int J Surg Case Rep. 2016; 28: 65-67.
7- P. G. M. Figueira, M. S. Abr˜ao, G. Krikun, and H. Taylor, “Stem cells in endometrium and their role in the pathogenesis of endometriosis,” Annals of theNew York Academy of Sciences, vol. 1221, no. 1, pp. 10–17, 2011.
8- I. Brosens, S. Gordts, and G. Benagiano, “Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion,” Human Reproduction, vol. 28, pp. 2026–2031, 2013.
9- Pinkert TC, Catlow CE, Straus R. Endometriosis of the urinary bladder in a man with prostatic carcinoma. Cancer 1979;43: 1562‑7.
10- Fukunaga M. Paratesticular endometriosis in a man with a prolonged hormonal therapy for prostatic carcinoma. Pathol Res Pract 2012; 208:59‑61.
11- Simsek G, Bulus H, Tas A, Koklu S, Yilmaz SB, Coskun A. An unusual cause of inguinal hernia in a male patient: Endometriosis. Gut Liver 2012; 6:284‑5.
12- Pankratjevaite L, Samiatina-Morkuniene D. A case report of thoracic endometriosis - A rare cause of haemothorax. Int J Surg Case Rep. 2017;33: 139-142.
13- Nair SS, Nayar J. Thoracic Endometriosis Syndrome: A Veritable Pandora's Box. J Clin Diagn Res. 2016 Apr;10(4): QR04-8.
14- P. Gruenwald, “Origin of endometriosis from the mesenchyme of the celomic walls,” American Journal of Obstetrics and Gynecology, vol. 44, no. 3, pp. 470–474, 1942.
15- S. Gupta, A. Agarwal, N. Krajcir, and J. G. Alvarez, “Role of oxidative stress in endometriosis,” Reproductive BioMedicine Online, vol. 13, no. 1, article 2291, pp. 126–134, 2006.
16- A. Augoulea, A. Alexandrou, M. Creatsa, N. Vrachnis, and I. Lambrinoudaki, “Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress,” Archives of Gynecology and Obstetrics, pp. 1–5, 2012.
17- Signorile PG, Baldi F, Bussani R, Viceconte R, Bulzomi P, D'Armiento M, D'Avino A, Baldi A. Embryologic origin of endometriosis: analysis of 101 human female fetuses. J Cell Physiol. 2012 Apr;227(4):1653-6.
18- R. O. Burney and L. Giudice, “Pathogenesis and pathophysiology of endometriosis,” Fertility and Sterility, vol. 98, pp. 511–519, 2012.
19- F. R. Oliveira, C. D. Cruz, H. L. del Puerto, Q. T. M. F. Vilamil, F. M. Reis, and A. F. Camargos, “Stem cells: are they the answer to the puzzling etiology of endometriosis?” Histology and Histopathology, vol. 27, no. 1, pp. 23–29, 2012.
20- H. Masuda, Y. Matsuzaki, E. Hiratsu et al., “Stem cell-like properties of the endometrial side population: implication in endometrial regeneration,” PLoS ONE, vol. 5, no. 4, Article ID e10387, 2010.
21- Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S,
Gordon SD, Henders AK, Martin NG, Attia J, Holliday EG, McEvoy M, Scott RJ,
Kennedy SH, Treloar SA, Missmer SA, Adachi S, Tanaka K, Nakamura Zondervan KT, Zembutsu H, Montgomery GW. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012 Dec;44(12):1355-9.
22- Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod. 2014 Jul;20(7):591-8.
23- Brosens I, Puttemans P, Benagiano G. Endometriosis: a life cycle approach? Am J Obstet Gynecol 2013 Mar 15.
24- Brosens I, Brosens J, Benagiano G. Neonatal uterine bleeding as antecedent of pelvic endometriosis. Hum Reprod. 2013 Nov;28(11): 2893-7.
25- Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007 Aug;25(8):2082-6.
26- Zhou Y, Gan Y, Taylor HS. Cigarette smoke inhibits recruitment of bonemarrow-
derived stem cells to the uterus. Reprod Toxicol 2011; 31:123–127.
27- Santulli P, Marcellin L, Menard S, Thubert T, Khoshnood B, Gayet V, Goffinet F, Ancel PY, Chapron C. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum Reprod. 2016 May;31(5):1014-23.
28- Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014 Sep;44(3):261-78.
29- Bulun SE. Endometriosis. N Engl J Med. 2009; 360:268–279.
30- Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, Lessey BA, Tayade C. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016
Apr;105(4): 968-977.e5.
31- Lanfrancone L, Boraschi D, Ghiara P, Falini B, Grignani F, Peri G, Mantovani A, Pelicci PG. Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage-CSF, interleukin-1 [IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood. 1992 Dec 1;80(11):2835-42.
32- Topley N, Mackenzie RK, Williams JD. Macrophages and mesothelial cells in bacterial peritonitis. Immunobiology 1996;195: 563–573.
33- Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, Witz CA. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril 2008;90: 1487–1495.
34- Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013 Jul-Aug;19(4):406-18.
35- Kohl Schwartz AS, Wölfler MM, Mitter V, Rauchfuss M, Haeberlin F, Eberhard M, von Orelli S, Imthurn B, Imesch P, Fink D, Leeners B. Endometriosis, especially
mild disease: a risk factor for miscarriages. Fertil Steril. 2017 Nov;108(5):806-814.e2.
36- Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum Reprod. 2014 Mar;29(3):462-72.
37- AlKudmani B, Gat I, Buell D, Salman J, Zohni K, Librach C, Sharma P. In Vitro
Fertilization Success Rates after Surgically Treated Endometriosis and Effect of
Time Interval between Surgery and in Vitro Fertilization. J Minim Invasive
Gynecol. 2017 Aug 12. pii: S1553-4650(17)31082-8.
38- Słabuszewska-Jóźwiak A, Ciebiera M, Baran A, Jakiel G. Effectiveness of laparoscopic surgeries in treating infertility related to endometriosis. Ann Agric Environ Med. 2015;22(2):329-31.
39- Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J Immunol. 2015 Sep 15;195(6):2591-600.